Evaluation of Pesticide Safety
CSS supports the EPA Office of Pesticide Programs in evaluating the safety and potential health and environmental risks resulting from the use of pesticides and ensures that the data utilized for EPA’s regulatory decisions are scientifically sound.

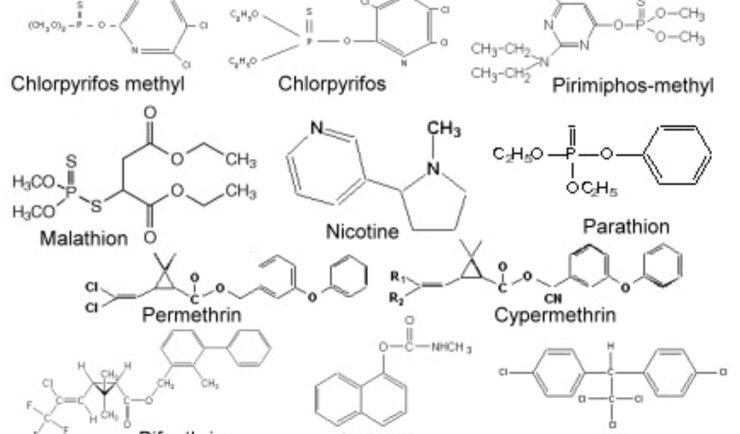
The Issue:
To ensure a plentiful and safe food supply and the control human and animal pests , the EPA is charged under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) with registering pesticides in the U.S. and ensuring that their use poses limited risks to human health and the environment.

The Solution:
To support the registration and use of pesticides in the U.S., agrochemical companies are required to submit numerous studies examining the toxicity of the pesticides to mammals and other non-target species, along with studies examining the fate (movement, degradation, metabolism) of pesticides in the environment. As these studies form the basis for EPA’s regulatory decisions, they must be independently evaluated to ensure that they are scientifically sound and conducted in accordance with current regulatory standards. CSS scientists provide EPA and independent and unbiased review of this data.

CSS Innovations and Contributions:
For more than 35 years covering multiple concurrent contracts, CSS scientists have supported EPA/OPP in the review and evaluation of the wide array of studies supporting pesticide registrations. To support human health risk assessments, our scientists review and evaluate studies pertaining to pesticide toxicity in model mammalian species in order to estimate potential toxicity to humans. These studies include both short- and long-term studies examining general toxicity, potential carcinogenicity, and reproductive effects. We evaluate studies examining potential pesticide residues in food and livestock commodities that serve as the basis of establishing allowable tolerances and estimating dietary exposure. To support environmental risk assessments, we also review studies examining toxicity to non-target plant and animal species, as well as, the movement and fate of pesticides in the environment. For all these reviews, our scientist prepare concise reports evaluating the study’s procedures, results and conclusions, and its adequacy in fulfilling regulatory requirements.
Key Technical Services
- Evaluation of pesticide residues in food/feed crops and livestock
- Evaluation of mammalian toxicity data for pesticides
- Determination of critical end-points, NOAELs and LOAELs (no- and lowest-observed adverse effect level), RfDs and RfCs (acceptable reference doses and concentrations), and cancer values
- Risk assessment and Weight of Evidence (WoE) analyses.
- Evaluation of chemical toxicity to non-target species
- Movement, metabolism, and degradation of pesticides in the environment
- Evaluation of regulatory compliance and quality assurance

Additional Projects

Analysis for Coastal Operational Resiliency
CSS assisted with planning and executing a demonstration of three decontamination technologies on a USCG vessel contaminated with a benign surrogate for anthrax.

Decontamination Field Tests for EPA
CSS staff helped design, plan for, and implement field tests of decontamination technologies and methods using MB for EPA’s Consequence Management Advisory Division.

Guiding Wildfire Response and Recovery
CSS supports communities following catastrophic wildfires to ensure that items discarded during site cleanup are properly identified, sorted, and sent to an appropriate disposal facility.

Get in touch
Contact us to learn more about our projects, capabilities, solutions, and service offerings.







